22 June 2020: Review Articles
Apoptosis in Autoimmunological Diseases, with Particular Consideration of Molecular Aspects of Psoriasis
Agata Krawczyk1EFG*, Joanna Miśkiewicz2EF, Karolina Strzelec2EF, Dominika Wcisło-Dziadecka3EF, Barbara Strzalka-Mrozik2EFDOI: 10.12659/MSM.922035
Med Sci Monit 2020; 26:e922035
Abstract
ABSTRACT: Apoptosis is a natural physiological process involving programmed cell death. Thanks to this process, it is possible to maintain the homeostasis of the body and the immune system. Dysfunctions of this mechanism lead to development of autoimmune diseases such as psoriasis; these diseases are chronic and treatment is extremely difficult. In psoriasis (a skin disease), apoptosis disorders are manifested by keratinocyte proliferation dysfunction. Autoimmune diseases coexisting with psoriasis include multiple sclerosis, autoimmune thyroid disease, and diabetes, but the common pathogenesis of these diseases is not fully understood. Given the heterogenous nature and chronic and recurrent course of psoriasis, the selection of an effective therapeutic strategy is still a problem. This literature review was focused on the process of apoptosis as a factor in the development of autoimmune diseases, with particular emphasis on psoriasis. The work also includes a review of therapeutic methods of psoriasis based on the latest literature.
Keywords: Autoimmune Diseases, Psoriasis, Apoptosis Regulatory Proteins, Diabetes Mellitus, Type 1, Graves Disease, Hashimoto disease, Inflammatory Bowel Diseases, Multiple Sclerosis
Apoptosis
INTRINSIC TRIAL:
The intrinsic apoptosis pathway involves one of the main intracellular organelles, the mitochondria. The internal pathway of programmed cell death can be activated as the result of an increase in the concentration of Ca2+ ions in the cytoplasm, DNA damage, an increase in the level of reactive oxygen species, oxidative stress, and electron transport disorders [5].
An important protein involved in the stimulation of proapoptotic factors is the p53 protein [5,6]. In addition, mitochondrial mega-channels, referred to as the permeability transition pore (PTP), occurring at the contact point of mitochondrial membranes play an important role. The main element of PTP is the adenine nucleotide translocator (ANT). In the inner membrane, the ANT mitochondrion forms a complex with a voltage-dependent anion channel (VDAC) and a benzodiazepine peripheral receptor (BRP). From the cytosolic side of the Bcl-2 family of proteins, and on the mitochondrial side of cyclophilin D, as well as creatine kinase (CK), glycerin (GK), and hexokinase (HK), can bind to the PTP channel [5,7].
The PTP mega-channel opens as the result of induction by proapoptotic factors. One of the factors that are transported by PTP to the cytoplasm is cytochrome c (Apaf-2), which binds to Apaf-1 through its C-terminal WD multiple repeat domain. With the participation of WD, dATP/ATP also joins the Apaf-1 structure. Apaf-1 connects to procaspase 9 through the N-terminal domain of caspase activation and recruitment domains (CARD). As a result of the hydrolysis of the ATP or dATP molecule, the so-called Walker cassettes (nucleotide-binding regions) are created. In subsequent stages, the oligomerization of 7 Apaf-1 particles occurs and thus the formation of a heptameric structure called the “death wheel” or apoptosome is created [8]. The resulting complex affects the activation of caspase 9, which in turn leads to activation of subsequent executive caspases. As a result of the processes taking place, protein proteolysis, morphological changes, and, ultimately, cell death occur [9].
EXTRINSIC TRIAL:
The extrinsic pathway of the apoptosis process is otherwise referred to as the receptor pathway. Its induction begins by joining a specific ligand with one of the membrane death receptors, which is a transmembrane molecule. Their extracellular fragments are characterized by the ability to bind ligands. In contrast, cytoplasmic domains are terminated with death receptors (DD), whose function is to transmit signals into the cell [10,11].
The largest group of death receptors is the TNFR superfamily, which includes Fas, CD40 (cluster of differentiation 40), and TNF-like weak inducer of apoptosis (TWEAK). The characteristic ligands binding to them are, respectively, tumor necrosis factor (TNF-α), Fas ligand (FasL), CD40 ligand (CD40L), and TNF-related apoptosis-including ligand (TRAIL) [10,12]. Under the influence of an appropriate protein, a protein-receptor combination occurs. These interactions induce the transmission of death signals, which then migrate to the death domain by activating procaspase 8. This process results in a death-inducing signaling complex (DISC) that converts procaspase 8 into the active TNF-R1 enzyme. When combined with TNF-α, it joins the TNF-R1-associated death domain (TRADD) [10,11]. The final stage of the extrinsic pathway of apoptosis is activation of the cascade of executive caspases (i.e., executive proteins) [12]. The receptor pathway can also bind to the mitochondrial pathway using the Bid protein [13].
PROTEINS FROM THE IAP FAMILY:
Proteins from the family of apoptosis inhibitors (IAPs) play an essential role in regulating the process of apoptosis. IAPs are made of chains with a structure of 150–1500 amino acids with 2 characteristic domains: the baculoviral IAP-like repeats (BIR) domain at the N-terminus and the really interesting new gene (RING) domain located at the C-terminus of the molecule, having the structure of a zinc finger [14]. Affiliation with IAP proteins is associated with the presence of the BIR domain. BIR domains are made up of about 70 amino acid residues that can occur in some proteins, even in triplicate. The RING zinc finger (RZF) domain has E3 ligase activity ubiquitin, which is a protein that plays a role in protein ubiquitination and IAP autoubiquitination [15].
Eight known IAP proteins are divided into 3 classes based on the homology of the BIR domains and the presence or absence of the RZF domain. The first class includes the following proteins: X chromosome-binding IAP (XIAP), cellular inhibitor of apoptosis 1 (cIAP1), cellular inhibitor of apoptosis 2 (cIAP2), IAP-like protein 2 (ILP-2), and livin (BIRC7). The XIAP polypeptide contains 3 BIR domains, and the linker region between BIR1 and BIR2 creates a spatial obstacle covering the active sites of caspases 3 and 7 [15]. In addition, the BIR3 domain has the ability to inhibit the formation of active caspase 9 homodimer [16]. XIAP proteins can also inhibit apoptosis in the caspase-independent pathway through activation of mitogen-activated protein kinases (JNK), which subsequently leads to phosphorylation of mitogen-activated protein kinase (MAP kinase) and thereby activation of NF-κB. In the structure of cIAP1 and cIAP2 proteins, the CARD caspase recruiting domain exists between the BIR and RZF domains. These proteins have the ability to inhibit apoptosis by blocking caspase 3 and 7, as well as at the level of membrane receptors by binding to TRAF1 and TRAF2 [17]. Livin and ILP-2 are proteins distinguished by the presence of only 1 BIR domain [15]. Livin inhibits apoptosis at both the mitochondrial and receptor levels by blocking caspase 3 and 7, as well as by inhibiting the active form of caspase 9 [18]. In vitro, ILP-2, like the livin protein, has the ability to interact with caspase 9 and the Bax protein. Under physiological conditions, such relationships are not found due to the high instability of this protein [15,19].
The second class of IAP proteins includes only the neuronal apoptosis inhibitory protein (NAIP), which is structurally characterized by the presence of 3 BIR domains in the absence of the RZF domain. The C-terminal structure of NAIP contains the oligomeric nucleotide-binding oligomerization domain (NOD) with leucine-rich repeats (LRRs). Due to the presence of LRRs, proteins containing this structure are able to bind intracellular lipopolysaccharides secreted by bacteria. In addition, these proteins are involved in the production of cytokines and the activation of NF-κB [15].
The third class of IAP proteins includes survivin and the BIR repeat containing ubiquitin-conjugating enzyme (BRUCE protein/Apollon.) These proteins are characterized by the presence of only 1 BIR domain and the absence of the RZF domain. Survivin shows, among others, the ability to inhibit caspase 9. The participation of survivin in the regulation of apoptosis via the cyclin-dependent p21WAF1/Cip1 kinase inhibitor and indirect inhibition of caspase 3 is also indicated. There are other mechanisms of survivin anti-apoptotic activity, and it has been shown that inhibition of survivin activity leads to DNA fragmentation due to apoptosis-inducing factor translocation (AIF) [20]. Survivin can also inhibit cell passage through the cell cycle phases G1 and S [21]. The second protein in the third class of IAP is the large BRUCE membrane protein, which has ubiquitin ligase E3 activity due to the presence of the Ub-binding domain UBC at the C-terminus. It exhibits an anti-apoptotic effect by inhibiting caspase, but its main action is based on ubiquitination and proteasome degradation of the high temperature requirement A2 (HtrA2)/Omi serine protease, caspase 9, and second mitochondria-derived activator of caspase/direct IAP binding protein with low Pi (Smac/DIABLO) [22].
Apoptosis and Autoimmune Diseases
The main task of a properly functioning immune system is to defend the body and maintain tissue homeostasis, using antigen control of both exogenous and endogenous origin. Autoimmune diseases are a group of diseases in etiopathogenesis which are particularly attributed to mediation of the immune system. In addition, a combination of environmental and molecular factors plays an important role in their induction, and autoimmunity is the mechanism underlying this process [1].
Epidemiological studies have shown that autoimmune diseases are an increasingly serious problem in modern society. However, their common pathogenesis is not completely understood. Autoreactive Th1 and Th17 lymphocytes play an important role in the development of these diseases, resulting from the loss of immunotolerance and autoantibodies directed against the structural elements of the body. Disorders of apoptosis also contribute to the development of autoimmune diseases [23].
The most common autoimmune diseases are psoriatic arthritis, rheumatoid arthritis, connective tissue diseases, multiple sclerosis, autoimmune thyroid diseases, celiac disease, and inflammatory bowel disease (Figure 2). Most autoimmune diseases affect women more often than men. In addition, the appearance of one disease from auto-aggression significantly increases the risk of the parallel development of another [23].
Autoimmune Thyroid Disease
TYPE I DIABETES:
Another autoimmune disease in which the pathological process takes place within the organs of the endocrine system is type I diabetes. In its course, the phenomenon of apoptosis contributes to the destruction of β-islet cells responsible for insulin production. Thus, this results in a decrease in their cell mass. Manifestation of the disease begins when the mass of β cells is reduced to 80% to 90%. In this case, it is impossible to maintain normal blood glucose levels in a sick person. However, effective treatment contributes to the renewal of β cells, leading to disease remission [30].
Hyperglycemia arising in the course of type I diabetes has an impact on immunological and metabolic processes as well as oxidative stress. An increase in glucose causes toxic effects on pancreatic islet β cells. This is done through the proapoptotic protein Bax, causing the release of cytochrome c from the mitochondrial space and activation of caspase 3. Therefore, their apoptosis is induced. The clinical consequence is a decrease in glucokinase expression and ATP synthesis, which causes a weakening of secretion and a decrease in insulin concentration in patients’ blood [30,31].
At the same time, antioxidative mechanisms dysfunction with an increase in reactive oxygen species. This phenomenon contributes to the peroxidation of lipids, proteins, and nucleic acids. The end result is the destruction of pancreatic islet β cells. Therefore, the introduction of compounds from the group of antioxidants as one of the possible therapeutic schemes contributes to the protection of Langerhans island cells against oxidative stress. Nicotinamide is an example of such a compound. However, based on the results of in vitro studies performed by Hoorens, no “protective” effect of the above compound against proinflammatory cytokine-dependent apoptosis of pancreatic β cells has been demonstrated, and only the effect on necrosis was noticed [32].
Apoptosis is also associated with other pathological processes in the course of diabetes. Leukoembolization is a pathological phenomenon in diabetes microcirculation consisting of the occurrence of an embolism. Protein glycosylation of cell membranes causes granulocytes to lose their ability to deform. This process is accompanied by an increase in IL-8 production and endothelial cell apoptosis [33].
Another situation concerns brain changes in the course of diabetes; neutrophilic factors are involved in the pathogenesis of these changes. In addition, mention should be made of the anti-apoptotic factor insulin-like growth factor 1 (IGF-1), which affects neuronal survival. Other inflammatory factors may additionally cause IGF-1-infected signal transduction disorders [34].
NON-SPECIFIC INFLAMMATORY BOWEL DISEASE:
Apoptosis disorders are also observed in the course of inflammatory bowel disease (IBD). The group of diseases includes ulcerative colitis, Crohn’s disease, and unspecified colitis. A characteristic clinical feature of these diseases is extensive inflammatory changes accompanied by abdominal pain. Their pathogenesis is not strictly defined, but a special role is assigned to the activity of genetic, environmental, and immunological factors. The most important genetic factor determining the occurrence of ulcerative colitis and Crohn’s disease is the family occurrence of given disease entities, for which the susceptibility genes are, respectively, IBD2 located on chromosome 12 and NOD2/CARD15, whose loci occurs on chromosome 16 [35].
In addition, dysfunctions in the process of apoptosis are one of the factors that induce disease in their course. Epithelial cells of the intestine undergo continuous regeneration as a result of the proliferation of structural units at the bottom of the intestinal crypts and programmed death at their top. This phenomenon conditions the proper renewal of the epithelium. Inflammatory bowel disease occurring in active form is characterized by an increase in the activity of the process of apoptosis and at the same time their proliferation. As a result of the imbalance between the above-mentioned processes, chronic, recurrent conditions develop [36].
Confirmation of this theory are the results of research carried out by Yukawa et al., suggesting an increase in the expression of apoptotic factors in the active form of ulcerative colitis. In Crohn’s disease, disturbances in the expression of genes encoding proteins from the Bcl-2 family participating in the mitochondrial pathway of apoptosis are observed. The expression of genes encoding the pro-apoptotics protein Bax and a high ratio of the anti-apoptotic protein Bcl-xL to Bax (Bcl-xL/Bax) is reduced. The result is apoptosis resistance, contributing to the development of a chronic disease process [36,37].
Inflammatory cytokines play an important role in the course of inflammatory bowel disease (IBD). Their role is to initiate the severity of the inflammatory process and to maintain it. This mainly involves IL-1, IL-6, and IL-23. There are many studies on the expression of proinflammatory cytokines in patients with IBD. The increased expression of these factors has been correlated with disease activity [38].
Studies have shown that the level of IL-6 expression is increased in patients with active ulcerative colitis, as well as in those with active and inactive forms of Crohn’s disease. According to the available literature, IL-6 activates the transcription factor STAT by attaching to the receptor. This stimulates the recruitment of T lymphocytes and granulocytes to the intestinal epithelium. In this way, the inflammatory process is initiated and the apoptosis of damaged cells is inhibited, thus resulting in tissue repair [39].
The inhibition of T cell apoptosis in the intestinal mucosa also plays an important role. As a result of this process, there is a loss of control over the cellular immune response, which is predominant in Crohn’s disease and humoral in the case of ulcerative colitis. As a consequence, the inflammatory process is constantly maintained in the intestinal wall. In addition, in ulcerative colitis an increase in Ki-67 antigen expression in intestinal crypt cells and a positive correlation with epithelial dysplasia have been shown. Determination of PCNA, or testicular proliferation antigen, is helpful in detecting foci of dysplasia of this disease. In Crohn’s disease, increased proliferation of intestinal crypt cells predisposes mucosa to mutation. Research conducted by Dandrieux et al. was based on the evaluation of proapoptotic markers Casp3 and PARP, as well as the anti-apoptotic Bcl-2. However, the literature related to the proliferative index in the course of IBD, presenting the results of the study, is insufficient [36,40].
MULTIPLE SCLEROSIS:
Multiple sclerosis (MS) is a progressive disease of the central nervous system, characterized by extensive demyelinating lesions resulting from abnormal apoptosis. The clinical picture of MS is manifested by changes in the central nervous system (CNS) occurring in the form of a neurological deficit. It is characterized by damage to the myelin sheath of the axons within the brain and spinal cord with associated inflammatory foci. The etiopathogenesis of this disease is extremely complicated. Genetic background as well as a significant contribution of the immune system are mentioned among the potential pathogenetic factors. Autoreactive T lymphocytes, proinflammatory cytokines, and regulatory cells, which are immune components that induce disease, play a special role in the development of MS. Viruses or bacteria belong to the group of environmental factors [41].
The main class II histocompatibility system (HLA class II system) is a genetic factor that is actually associated with the induction of a pathological process. Susceptibility to MS development is determined by the HLA DRI 501, HLA DR 4, and HLA DP5 alleles, depending on the population in which they occur. In addition, genes such as MBP, ApoE, ICAM1, PTPRC, and MHC2TA, and also the CARD11 gene associated with the process of apoptosis, are significantly involved in the development of MS [42].
The most likely mechanism leading to the degeneration of nerve cells is their programmed death process induced by calcium ion-dependent enzymes. As a result of an increase in the concentration of Ca2+ ions in the neuronal cytoplasm, i.e. the overload arising due to disruption of the ion channels in the cell membrane, a cascade of reactions leading to the process of apoptosis begins. This situation occurs, among others, in the case of hypoxia of nerve cells during ischemic stroke or as a result of inhibition of neuronal nutrition due to the destruction of oligodendrocytes by an ongoing inflammatory process. In the case of MS, neuronal hypoxia in inflammatory foci may also occur as a result of the production of large amounts of nitric oxide (NO), causing disturbances in ATP production [43].
An increase in the concentration of calcium ions in the cytoplasm induces proteolytic and lipolytic enzymes and protein kinases. As a result of simultaneous activation of the aforementioned enzymes and nitric oxide synthetase, damage occurs, and as a consequence, so does neuron death. The conduction of nerve impulses in damaged nerve cells leads to an increase in the use of cellular energy compared to a neuron with a normal structure. Thus, along with the accompanying mitochondrial dysfunction caused by the action of nitric oxide, a condition called “virtual hypoxia”, calcium disorders, and nerve cell damage are introduced [44].
The direct pathway of neurodegeneration in MS and experimental autoimmune encephalomyelitis (EAE) is based on damage to nerve cells as a result of their contact with T lymphocytes and monocytes. CD4+ and CD8+ lymphocytes infiltrate the CNS. When they come into contact with the body of the neuron or its projections, a cascade of reactions leading to cell death is initiated. As a result of the interaction of the death ligand with a specific receptor, which can be expressed on the surface of nerve cells, oligodendrocytes, and autoreactive T cells, cell apoptosis occurs. As a result of the ligand interaction with the death receptor, intracellular transmission based on the presence of molecules Fas-associated death domain (FADD), TNF receptor-associated death domain (TRADD), FLICE-like protein inhibitors (FLIP) activating caspase 8, 3, and 7. Ultimately, this leads to cell apoptosis [41,45].
Activated macrophages and microglia cells are characterized by the production of proinflammatory cytokines TNF-α, IL-1, and IL-6, which also have the ability to induce the process of nerve cell apoptosis. In addition, these cells in inflammatory foci increase the amount of oxygen free radicals (ROS), which are characterized by their high affinity for nucleic acid structures, thereby damaging neurons. As a result of ROS, cytochrome c and AIF are also released from mitochondria [45].
The indirect route of the neurodegeneration process in the course of MS is a consequence of the ongoing inflammatory process. Under the influence of antigen-specific myelin sheath T lymphocytes, phagocytosis occurs, along with the production of proinflammatory cytokines and reactive oxygen species by macrophages. Ultimately, the above-mentioned phenomena lead to the exposure of the nerve fiber [41].
The process of apoptosis is also discussed in the aspect of oligodendroglial cell death. In addition, Bcl-2-positive lymphocytes have been shown in MS plaques. Their number is greater in the stage of progressive disease and in the area of remyelination. Fas expression has been shown to be central to the death process of central nervous system astrocytes. The intensity of oligodendrocyte apoptosis is caused by overexpression of the p53 transcription factor. A similar situation applies to caspases [46].
PSORIASIS:
Psoriasis is an autoimmune disease in which both cellular and humoral responses are disturbed. Due to the autoimmune nature, particular attention is paid to the role of uncontrolled activation of autoreactive helper T cells differentiating from naïve T cells. There are 3 types of T lymphocyte formation: IL-12/Th1, IL-22/Th22, and IL-23/Th17 [23].
The inflammatory process underlying the development of the disease begins with the presentation of the autoantigen on antigen-presenting cells (APCs). The first stage of the disease can be initiated by various factors, including infectious agents, injuries, or stress. These factors have the ability to release cytokines, i.e. IL-1 and TNF-α, from keratinocytes, which in the next stage activate skin macrophages and dendritic cells (DCs). DCs migrating to the lymph nodes initiate T cell activation. This is a model of an immunological synapse integrating T cell signaling pathways. This combination leads to the induction of the IL-12/Th1/IFN-γ pathway. Interleukin 12 plays a key role in the differentiation of Th1 lymphocytes, which affects the growth of IFN-γ via the STAT4 receptor. In addition, interferon γ increases the activity of the leading transcription factor Th1, T-bet, thus affecting a further increase in the synthesis of interferon γ while reducing the synthesis of IL-4 and IL-5 [47].
IL-23 is another interleukin that plays an important role in the pathogenesis of psoriasis, which is produced by dendritic cells and other APCs that affect the development and maturation of Th17 effector lymphocytes. The process of maturation and differentiation occurs with the participation of transforming growth factor β (TGF-β), IL-1b, IL-6, and IL-21. In addition, IL-23 inhibits IL-22 synthesis [48]. Thus, IL-23 has an activating effect on the Th17/Il-23 axis [49]. The main cytokines produced by Th17 lymphocytes are IL-6, IL-17, IL-21, and IL-22. Interleukin 21 re-stimulates Th17 lymphocyte synthesis. Interleukin 17A stimulates the production of IL-6, IL-8, and G-CSM by endothelial cells, epithelial cells, and keratinocytes. In addition, proliferation of keratinocytes has been shown to be stimulated by IL-22, and the severity of the changes correlates with IL-23 and IL-17. It is known, therefore, that the immune response initiated by Th1 under the influence of IL-12 is maintained by IL-23, which stimulates Th17 maturation and activity and also affects the maintenance of an appropriate pool of memory cells [47].
Another Th lymphocyte population significantly increased in psoriasis is the Th-22 lymphocyte population promoted by IL-6, TNF-α, and plasmacytoid dendritic cells. Th-22, Th-17, and Th-1 lymphocytes have the ability to produce IL-22, whose roles in the pathogenesis of psoriasis have been confirmed in animal models. The Th22/IL22 axis is thus activated. IL-12 has the ability to differentiate keratinocytes and also regulates the expression of genes responsible for antibacterial defense. In addition, IL-22 interferes with the disruption of their physiological exfoliation by interfering with keratinocyte differentiation. IL-22 affects the extracellular degradation of connective tissue stroma in connection with the production of MMPs 1 and 3 (matrix metalloproteinases) by stimulating the production of chemokines and also plays a role in the accumulation of neutrophils in the skin. IL-22 also stimulates the production of IL-20 with a similar mechanism of action [49].
It has been shown that the second mechanism involved in the etiopathogenesis of psoriasis is a disorder of the humoral response based on the presence of autoreactive B lymphocytes responsible for the recognition of autoantigens without connection with major histocompatibility complex (MHC) molecules as well as the production of autoantibodies [49]. Psoriasis patients have been shown to have the presence of anti-nuclear antibodies (ANA) [50]. The specific autoantigen that triggers the autoimmune response is not known; however, several potential autoantigens are highlighted. Research conducted on bacterial superantigens with which skin autoantigens may cross-react have enabled the specification of the role of keratin 13, 16, and 17 (K13, K16, K17) and heterogeneous A1 ribonucleoprotein (hnRNP-A1) in the challenge of autoimmune response in patients with psoriasis. In addition, increased expression of keratin 6, 14, 16, and 17 and decreased expression of keratin 1 and 10 in psoriatic lesions have been shown. Their increased expression in in vitro stimulation by IL-1b and IFN-γ cytokines has been demonstrated [47]. K17 has also been shown to have an important role in the pathogenesis of psoriasis. IFN-γ, IL-17A, and IL-22 regulate keratin K17 mRNA and its concentration in keratinocytes. Another potential antigen that can trigger an autoimmune response is Pso p27 (psoriasis-associated antigen). Pso p27 occurs in diseased keratinocytes and lymphocytes and plays an important role in the activation of the complement system, and it participates in the formation of immune complexes and supports the inflammatory process by activating T lymphocytes [51].
It has also been proven that as a result of viral and bacterial infections, the immune response is strongly activated due to the structural similarity of the bacterial or viral epitope to the autoantigen. As a result of structural similarity, an autoimmune response may occur due to the molecular mechanism of mimicry [47]. It has been demonstrated that psoriatic lesions can also be caused by colonization and infection with pathogens from the Staphylococcus and Streptococcus groups. There is evidence that streptococcal groups A, C, and G infections are the provoking factor for psoriasis. As a result of the appearance of the M protein and peptidoglycan in the bloodstream, they are absorbed by monocytes or macrophages and then initiate autoimmunity. Homology between streptococcal protein M and type I keratin has been demonstrated. Psoriasis patients have a high titer of anti-peptidoglycan, which is a component of the streptococcal cell wall [52].
APOPTOSIS IN PSORIASIS:
Psoriasis is a disease in which there is a significantly reduced number of apoptotic cells compared to normal epidermis of people without dermatological dysfunction [10]. Wrone-Smith et al. proved that psoriatic keratinocytes are also resistant to factors that induce programmed cell death. As a result of keratinocyte resistance to proapoptotic signals transmitted by TNF-α, a paradoxical increase in the concentration of this proinflammatory cytokine occurs. Inhibitors of apoptosis include the survivin protein belonging to the IAP family that binds to caspases, virtually absent in the epidermis not affected by the disease process. However, the process of apoptosis, which is one of the main factors clearly affecting the pathomechanism of the disease, is not well understood.
It is known that the process of apoptosis is regulated by proteins from the Bcl-2 family, which include proapoptotic (Bax, Bak, Bad) and anti-apoptotic (Bcl-2, Bcl-xL) proteins. The earliest discovered protein belonging to this group is the anti-apoptotic Bcl-2 protein. All proteins of the family have at least one of the 4 regions of the so-called Bcl-2 homology domains in common: BH1, BH2, BH3, and BH4. Bcl-2 family proteins are divided by their function and structure. The first group includes anti-apoptotic proteins, i.e. Bcl-2, Bcl-xL, and MCL-1, and in their structure have all 4 BH domains and also a transmembrane signaling-anchoring sequence. Group 2 proteins are proapoptotic proteins having the BH1, BH2, and BH3 domains, i.e. Bax, Bak, and Bok. The third group includes proapoptotic proteins containing only the BH3 domain (Bim, Bik, Bnip3, Bnip3l, Noxa, Puma, Spike, and Hrk) (Figure 3) [53]. Studies by Takahashi et al. [54] showed increased expression of the anti-apoptotic Bcl-xL protein in psoriatic epidermis compared to epidermis without dermatological dysfunction. It is suggested that the increased expression of the gene encoding the anti-apoptotic Bcl-xL protein affects epidermal acanthosis in the course of the disease process. It has been shown that TNF-α increases the expression of the Bcl-xL gene as well as Bcl-2 and the gene encoding the Bax proapoptotic protein in psoriasis [54]. Understanding the mechanism of the effect of TNF-α on extending the life of epidermal cells has enabled the introduction of innovative treatment methods such as TNF-α inhibitors belonging to the group of biological drugs.
One of the stimuli initiating the process of apoptosis is the combination of the CD40 protein, which is a receptor belonging to the TNFR family with its ligand, CD40L. The CD40 molecule is a glycoprotein present on dendritic cells, macrophages, T and B lymphocytes, monocytes, basophils, epithelial cells, and endothelial cells. Expression of CD40 can be stimulated by the proinflammatory cytokines TNF-α, IL-1, IL-3, IL-4, and IFN-γ [55]. The CD40 ligand, also called CD40L or CD154, is a transmembrane protein that is a member of the TNF superfamily. CD40L is expressed mainly on activated T and B cells as well as monocytes, NK cells, mast cells, and basophils. The interaction between CD40 and CD40L triggers a signal contributing to the initiation of cellular immune responses.
The CD40/CD40L system is found in healthy skin. In psoriasis, both CD40 and CD40L expression are increased, with CD40L overexpression mainly occurring at the initial stage of psoriatic lesions. Myśliwiec et al. [10] reported in a review work a significantly higher concentration of CD40 and CD40L in the skin keratinocytes of patients with psoriasis in comparison with healthy skin keratinocytes [10]. These studies confirm that signals triggered by the CD40/CD40L system are important for regulating various signaling pathways, including NFκB factor pathways, mitogen-activated protein kinase pathways (MAPK), the 3-phosphatidylinositol 3-kinase pathway (PI3K), and the Cγ phospholipase pathway (PLCγ). Thus, various cellular processes, including the process of apoptosis, are regulated through these pathways [56].
The Fas receptor, a member of the TNF family, is thought to play an important role in the induction of psoriatic lesions. The Fas receptor ligand (FasL), which is a transmembrane protein, also belonging to the TNF family. As a result of the binding of the Fas receptor to FasL, apoptosis is induced. Research conducted by Takahashi et al. clearly indicates a significantly increased Fas expression in psoriatic keratinocytes compared to normal epidermis [54]. Myśliwiec et al. [57] showed in another study that the concentration of soluble Fas protein (sFas), which is considered an inhibitor of apoptosis in the serum of psoriasis patients, is significantly increased compared to the serum concentration of healthy individuals [57].
TUMOR NECROSIS FACTOR α AS A MAIN OF PROINFLAMMATORY CYTOKINE:
Tumor necrosis factor α is a cytokine involved in the inflammatory and immune responses. The genes for TNF-α are located among MHC genes, on chromosome 6p213. Acting through specific receptors, it initiates various reactions, including inflammatory reactions, septic shock, and apoptosis. Active TNF-α is a homotrimer. Tumor necrosis factor precursor is a transmembrane protein with a molecular weight of 26 kDa, while the TNF-α converting enzyme (TACE/ADAM 17) produces a free form with a molecular weight of 17 kDa. TNF-α is mainly produced by monocytes and macrophages and additionally by neutrophils, keratinocytes, mast cells, fibroblasts, endothelial cells, and cardiomyocytes. TNF-α can bind to 2 types of receptors: TNF-R1 found on most nuclear cells and TNF-R2 expressed on immune cells. In addition, IL-1, IFN-γ, IL-2, and TNF-α increase the expression of TNF-α receptors [58].
As a result of TNF-α attachment to the TNF-R1 receptor, there is a cleavage from the intracellular part of the so-called death domain of the protein SOD inhibitor, which leads to the formation of active protein trimers. In the next stage, the TRADD protein is activated, which forms complexes with the transmitters RIP, TRAF2, and FADD, activating subsequent stages of signal transduction, including the NF-κB-related pathway, the MAPK pathway, or the caspase-related pathway [58]. The TRADD-RIP-TRAF2 combination leads to activation of the κB inhibitor kinase (lκB), which releases in the cytoplasm of NF-κB through lκB phosphorylation. NF-κB affects the transcription of genes, including genes encoding anti-apoptotic proteins. Regulation of cell death via the TRADD-RIP-TRAF2 complex also occurs through the activation of JNK kinase and as a result of stimulation of the MAPK complex. In addition, the TRADD-RIP-FADD complex acts through caspase 8 activates caspase 3 and 7, thus ultimately affecting cell apoptosis [59].
Tumor necrosis factor affects cells of the immune system, causing many effects (Table 1) [60]. In psoriasis, TNF-κ has been shown to induce the proinflammatory cytokine synthesis by activated lymphocytes or keratinocytes. In addition, TNFα has been shown to increase the effects of NF-κB, which is a cytokine nuclear transcription factor, i.e. ICAM-1, VCAM-1, TNF-α, IL-6, and IL-8. As a result of the aforementioned activation of the NF-κB factor, the inflammatory reaction is strengthened and the process of keratinocyte apoptosis is abolished [61].
PSORIASIS TREATMENT AND NORMALIZATION OF THE APOPTOSIS PROCESS:
Psoriasis is characterized by hyperproliferation of keratinocytes with subsequent thickening of the epidermis, which results from impairment of the programmed cell death process. In recent years, the effect of various treatment methods used in psoriasis on the regulation of apoptosis and the restoration of normal keratinocyte homeostasis has become the subject of research. In one of the latest studies by Shi et al. concerning the effect of oxymatrine or acitretin treatment on cell proliferation and apoptosis in psoriatic epidermis, monoclonal antibodies directed against nuclear antigens (PCNA), anti-Ki antibodies were used as well as murine anti-Bcl-2 monoclonal antibodies. The Terminal Deoxynucleotidyl Transferase Mediated d-UTP Nick End-Labeling (TUNEL) method was also used. Researchers have shown that oxymatrine significantly reduces psoriatic lesions, which confirms the decrease in Psoriasis Activity and Severity Index (PASI) after treatment. In addition, a decrease in the proliferation index value indicates that oxymatrine inhibits epidermal cell proliferation. However, oxymatrine has been shown to inhibit cell apoptosis by increasing the expression of the anti-apoptotic Bcl-2 protein [62].
The improvement of normal homeostasis of psoriatic epidermal cells is mainly based on the restoration of the normal mechanism of cell apoptosis. Studies carried out in recent years with regard to biological drugs directed against TNF-α have shown their significant influence on the regulation of the activity of pro- and anti-apoptotic proteins. Studies carried out by Kokolakis et al. [63] showed a significant reduction in the expression of genes encoding anti-apoptotic proteins Bcl-2, Bcl-xL, and the transcription factor NF-κB, as well as an increase in the expression of genes encoding the proapoptotic proteins p53, Bax, and AIF in patients treated with infliximab. The observed changes in expression correlated with a decrease in epidermal thickness as well as a decrease in the value of surface indicators and the severity of psoriatic lesions, which indicates a positive effect of the use of TNF-alpha antagonists in patients with psoriasis [63]. Similar conclusions regarding the efficacy of biological drugs in the treatment of psoriasis were obtained by Yu et al. [64]. The authors examined 30 patients with moderate to severe plaque psoriasis treated only with etanercept or etanercept in combination with methotrexate (MTX). Researchers showed lower serum levels of interleukin (IL)-17A, IL-23, and tumor necrosis factor α, as well as a reduction in their mRNA expression in peripheral blood mononuclear cells (PBMCs) in patients undergoing combination therapy compared to patients undergoing monotherapy. However, the incidence of adverse effects was higher in patients receiving etanercept and methotrexate (60%) compared to patients taking only etanercept (33.3%). Therefore, the authors of these studies have demonstrated the effectiveness of etanercept in plaque psoriasis, as well as increased efficacy when methotrexate is included in treatment [62]. It has also been shown that methotrexate monotherapy significantly increases the expression of caspase 9 and decreases the expression of genes encoding Bcl-xL, c-FLIP, NFκBp65, and pAkt1, which indicates a beneficial effect of MTX on the induction of the mitochondrial apoptosis pathway, thus controlling acanthosis [65].
Conclusions
Apoptosis is a process of programmed cell death. It is a complex process in which many components are involved. The tumor necrosis factor family plays a special role in this process. Moreover, the Bcl2 family proteins, as regulators of this process, are equality important. Many autoimmune diseases are based on this aspect. One of them is psoriasis. Normalization of the apoptosis process in the course of psoriasis may lead to the reduction of clinical changes. For this purpose, a number of therapeutic methods are used, but biological drugs are the therapeutic future.
Figures
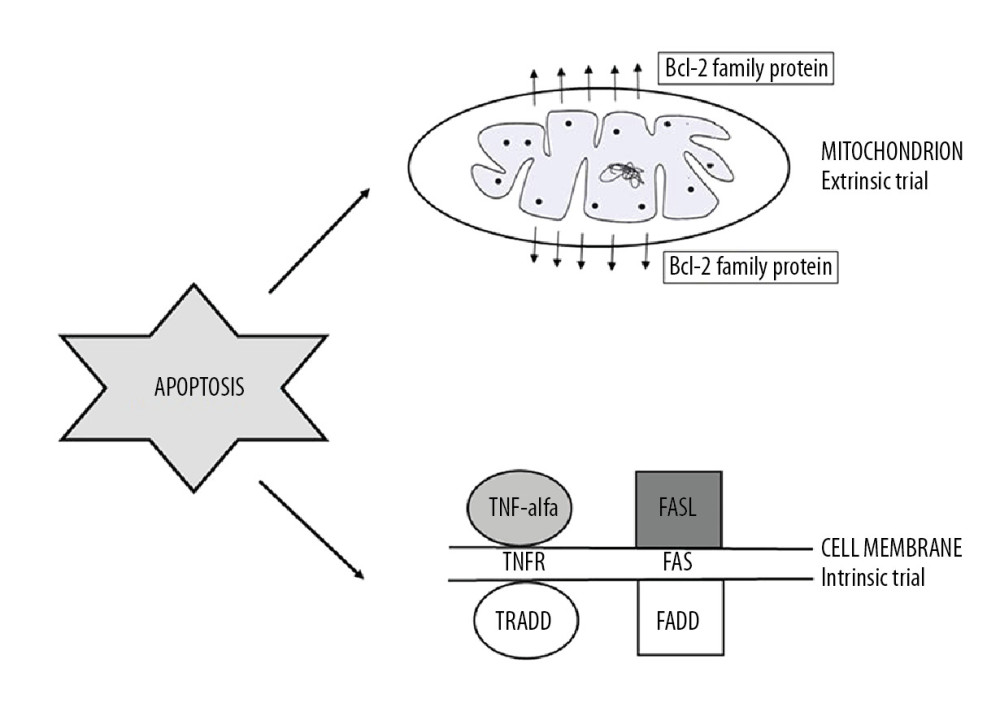 Figure 1. Apoptosis trials: intrinsic and extrinsic (TNF-alpha – tumor necrosis factor alpha; TNFR – tumor necrosis factor alfa receptor; FasL – Fas ligand; TRADD – TNFRSF1A Associated Via Death Domain; FADD – Fas-associated protein with death domain).
Figure 1. Apoptosis trials: intrinsic and extrinsic (TNF-alpha – tumor necrosis factor alpha; TNFR – tumor necrosis factor alfa receptor; FasL – Fas ligand; TRADD – TNFRSF1A Associated Via Death Domain; FADD – Fas-associated protein with death domain). 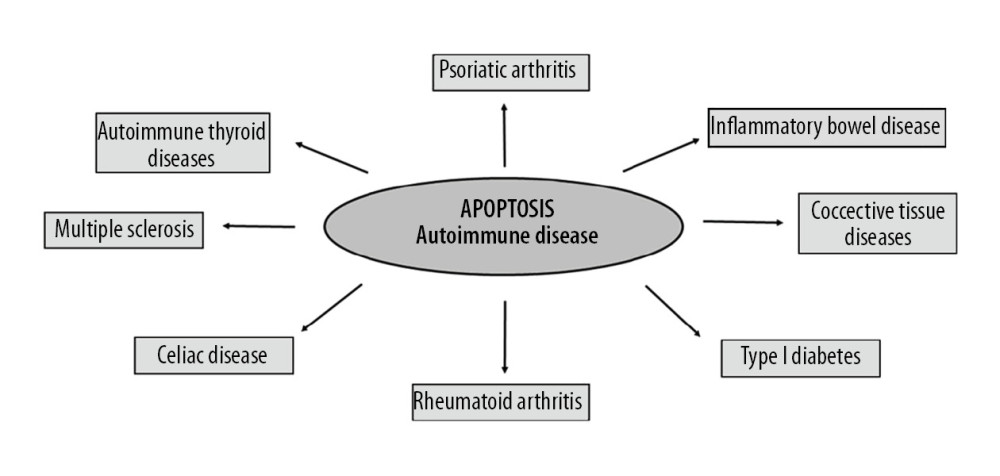 Figure 2. Autoimmune diseases based on apoptosis.
Figure 2. Autoimmune diseases based on apoptosis. 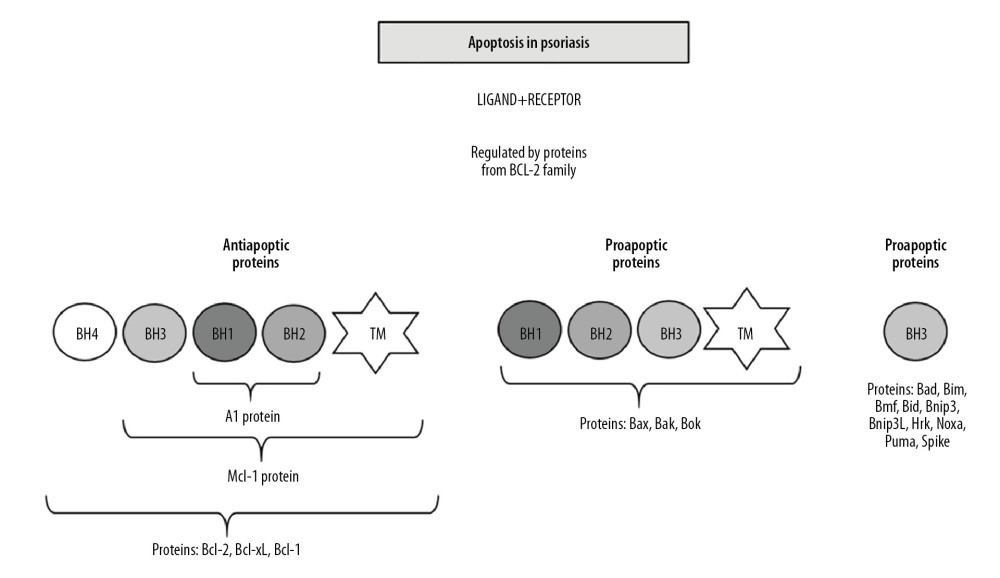 Figure 3. Apoptosis in psoriasis mainly regulated by Bcl-2 family proteins (BH1-BH4 – proteins domains; TM – transmembrane domain).
Figure 3. Apoptosis in psoriasis mainly regulated by Bcl-2 family proteins (BH1-BH4 – proteins domains; TM – transmembrane domain). References
1. Bossowski A, Czarnocka B, Stasiak-Barmuta AAnalysis of Fas, FasL and Caspase-8 expression in thyroid gland in young patients with immune and non-immune thyroid diseases: Endokrynol Pol, 2007; 58; 303-13 [in Polish]
2. Pereira WO, Amarante-Mendes GP, Apoptosis: A programme of cell death or cell disposal?: Scand J Immunol, 2011; 73; 401-7
3. Łącka K, Maciejewski AThe role of apoptosis in the etiopathogenesis of autoimmune thyroiditis: Pol Merk Lek, 2012; 18; 87-92 [in Polish]
4. De Bruin EC, Medema JP, Apoptosis and non-apoptotic deaths in cancer development and treatment response: Cancer Treat Rev, 2008; 34; 737-49
5. Łabędzka K, Grzanka A, Izdebska MMitochondria and cell death: Post Hig Med Dośw (online), 2006; 60; 439-46 [in Polish]
6. Yu J, Zhang L, The transcriptional targets of p53 in apoptosis control: Biochem Biophys Res Commun, 2005; 331; 851-58
7. Kroemer G, Mitochondrial control of apoptosis: An introduction: Biochem Biophys Res Commun, 2003; 304; 433-35
8. Scorrano L, Ashiya M, Buttle K, A distinct pathway remodels mitochondrial cri-stae and mobilizes cytochrome c during apoptosis: Dev Cell, 2002; 2; 55-67
9. Baliga B, Kumar S, Apaf-1/cytochrome c apoptosome: An essential initiator of caspase activation or just a sideshow?: Cell Death Differ, 2003; 10; 16-18
10. Myśliwiec H, Baran A, Flisiak ISelected aspects of apoptosis in psoriasis: Przegl Dermatol, 2017; 104; 57-63 [in Polish]
11. Zapolska-Downar D, Sygitowicz G, Jarosz MRole of apoptosis in the pathogenesis of artherosclerosis: Kardiol Pol, 2008; 66; 347-57 [in Polish]
12. Kastelan M, Pripic-Massari L, Brajac I, Apoptosis in psoriasis: Acta Dermatovenereol Croat, 2009; 17; 182-86
13. Raj D, Brash DE, Grossman D, Keratinocyte apoptosis in epidermal development and disease: J Invest Dermatol, 2006; 126; 243-57
14. Grzybowska-Izydorczyk O, Smolewski PThe role of the inhibitor of apoptosis protein (IAP) family in hematological malignancies: Post Hig Med Dosw (online), 2008; 62; 55-63 [in Polish]
15. Grzybowska-Izydorczyk O, Smolewski PInhibitor of apoptosis protein (IAP) and their antagonists: the role in cell biology and potential significance for carcinogenesis or targeted antitumor treatment: Acta Haematologica Polonica, 2009; 40(3); 593-612 [in Polish]
16. Sanna MG, da Silva Correia J, Ducrey O, IAP suppression of apoptosis involves distinct mechanisms: The TAK1/JNK1 signaling cascade and caspase inhibition: Mol Cell Biol, 2002; 22; 1754-66
17. Choi J, Hwang YK, Sung KW, Expression of livin, an antiapoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemia: Blood, 2007; 109; 471-77
18. Kasof GM, Gomes BC, Livin, a novel inhibitor of apoptosis protein family member: J Biol Chem, 2001; 276; 3238-46
19. Shin H, Renatus M, Eckelman BP, The BIR domain of IAP-like protein 2 is conformationally unstable: implications for caspase inhibition: Biochem J, 2005; 385; 1-10
20. Fukuda S, Mantel CR, Pelus LM, Survivin regulates hematopoietic progenitor cell proliferation through p21WAF1/Cip1-dependent and -independent pathways: Blood, 2004; 103; 120-27
21. Bratke T, Pohl C, Pyrowolakis G, Jentsch S, Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase: Mol Cell, 2004; 14; 1801-11
22. Sekine K, Hao Y, Suzuki Y, HtrA2 cleaves Apollon and induces cell death by IAP-binding motif in Apollon-deficient cells: Biochem Biophys Res Commun, 2005; 330; 279-85
23. Owczarczyk-Saczonek A, Placek W, Psoriasis as an autoimmune disease: Przegl Dermatol, 2014; 101; 278-87
24. Zagrodzki P, Kryczyk J, Znaczenie selenu w leczeniu choroby Hashimoto: Post Hig Med Dosw (online), 2014; 68; 1129-37 [in Polish]
25. Łącka K, Maciejewski M, Współczesne poglądy na temat etiopatogenezy autoimmunologicznego zapalenia tarczycy (choroby Hashimoto): Pol Merk Lek, 2011; 30; 132-38 [in Polish]
26. Chen S, Fazle Akbar SM, Zhen Z, Analysis of the expression of Fas, FasL and Bcl-2 in the pathogenesis of autoimmune thyroid disorders: Cell Mol Immunol, 2004; 3; 224-28
27. Wang SH, Van Antwerp M, Kuick R, Microarray analysis of cytokine activation of apoptosis pathways in the thyroid: Endocrinology, 2007; 148; 4844-52
28. Choi BM, Pae HO, Jang SI, Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator: J Biochem Mol Biol, 2002; 35; 116-26
29. Feldkamp J, Pascher E, Schott M, Soluble Fas is increased in hyperthyroidism independent of the underlying thyroid disease: J Clin Endocrinol Metab, 2001; 86; 4250-53
30. Niedźwiecki P, Zozulińska-Ziółkiewicz DClinical remission of type 1 diabetes: Diabetol Klin, 2013; 2; 185-90 [in Polish]
31. Han D, Yang B, Claycombe KJ, Glucotoxicity-induced apoptosis and suppression of cell proliferation in pancreatic beta-cells: FASEB J, 2008; 22; 1095
32. Garcia-Ocaña A, Alonso LC, Glucose mediated regulation of beta cell proliferation: The Open Endocrinology Journal, 2010; 4; 55-65
33. Massa R, Vale C, Macedo M, Furtado MJ, Purtscher-like retinopathy: Case Rep Ophthamol Med, 2015; 2015 421329
34. Candeias E, Sebastiao I, Vardoso S, Carvalho C, Brain GLP-1/IGF-1 signaling and autophagy mediate exendin-4 protection against apoptosis in type 2 diabetic rats: Mol Neurobiol, 2018; 55(5); 4030-50
35. Bartnik W, Wytyczne postępowania w nieswoistych chorobach zapalnych jelit: Prz Gastroenterol, 2007; 2; 215-29 [in Polish]
36. Rychlik A, Nowicki M, Szweda M, Kaczmar EExpression of Ki-67, PCNA markers and the apoptotic index in the inflammatory bowel disease in dogs and its diagnostic values: Med Weter, 2017; 73; 567-71 [in Polish]
37. Di Sabatino A, Ciccocioppo R, Luinetti O, Increased enterocyte apoptosis in inflamed areas of Crohn’s disease: Dis Colon Rectum, 2003; 46; 1498-507
38. Neurath MF, Cytokines in inflammatory bowel disease: Nat Rev Immunol, 2014; 14(5); 329-42
39. Katsans KH, Papadakis KA, Inflammatory bowel disease: Updates on molecular targets for biologics: Gut Liver, 2017; 11(4); 455-65
40. Markova AA, Kashkina EI, Maslyakova GNNew opportunities in the diagnosis of ulcerative colitis: Eksp Klin Gastroenterol, 2015; 2; 35-39 [in Russian]
41. Woliński P, Jałosiński M, Głąbiński AMechanisms of neurodegeneration and its markers in multiple sclerosis: Aktualn Neurol, 2008; 8; 25-32 [in Polish]
42. Achiron A, Gurevich M, Friedman N, Blood transcriptional signatures of multiple sclerosis: Unique gene expression of disease activity: Ann Neurol, 2004; 55; 410-17
43. Stys PK, General mechanisms of axonal damage and its prevention: J Neurol Sci, 2005; 233; 3-13
44. Stys PK, Axonal degeneration in multiple sclerosis: Is it time for neuroprotective strategies?: Ann Neurol, 2004; 55; 601-3
45. Aktas O, Smorodchenko A, Brocke S, Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL: Neuron, 2005; 46; 421-32
46. Friese MA, Schattling B, Fugger L, Mechanisms of neurodegeneration and axonal dysfiction in multiple sclerosis: Nat Rev Neurol, 2014; 10(4); 225-38
47. Fallen RS, Mitra A, Morrisey L, Lima H, Psoriasis as a chess board – an update of psoriasis pathophysiology: Psoriasis – types, causes and medication, 2013; 57-90, Rijeka, Croatia, InTech
48. Murdaca G, Colombo BM, Puppo F, The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases: Intern Emerg Med, 2011; 6; 487-495
49. Gołąb J, Jakóbisiak M, Lasek W, Stokłosa T: Immunologia, 2013, Warszawa, Wydawnictwo Naukowe PZWL [In Polish]
50. Azarsiz E, Ertam I, Karaca N, IgG-anti-IgA antibodies: An autoimmune finding in patients with psoriasis vulgaris: Minerva Med, 2012; 103; 183-87
51. Iversen OJ, Lysvand H, Hagen L, The autoantigen Pso p27: A post-translational modification of SCCA molecules: Autoimmunity, 2011; 44; 229-34
52. Ghoreschi K, Laurence A, Yang XP, Hirahara K, O’Shea JJ, T helper 17 cell heterogeneity and pathogenicity in autoimmune disease: Trends Immunol, 2011; 32; 395-401
53. Rupniewska Z, Bojarska-Junak AApoptosis: Mitochondrial membrane permeabilization and the role played by Bcl-2 family proteins: Post Hig Med Dosw (online), 2004; 58; 538-47 [in Polish]
54. Takahashi H, Manabe A, Ishida-Yamamoto A, Aberrant expression of apoptosis-related molecules in psoriatic epidermis: J Dermatol Sci, 2002; 28; 187-97
55. Myśliwiec H, Flisiak I, Baran A, Evaluation of CD40, its ligand CD40L and Bcl-2 in psoriatic patients: Folia Histochem Cytobiol, 2012; 24; 75-79
56. Bishop GA, Moore CR, Xie P, TRAF proteins in CD40 signaling: Adv Exp Med Biol, 2007; 597; 131-51
57. Myśliwiec H, Baran A, Myśliwiec P, Upregulation of the sFas/sFasL system in psoriatic patients: Adv Med Sci, 2015; 60; 64-68
58. Lubecka-Macura A, Kohut MTNF superfamily – mechanisms of action, biologic functions and therapeutic possibilities: Gastroenterology Review, 2010; 5(6); 303-9 [in Polish]
59. Wang X, Lin Y, Tumor necrosis factor and cancer, buddies or foes?: Acta Pharmacol Sin, 2008; 29; 1275-88
60. Male D, Brostoff J, Roth DB: Immunologia, 2008, Wrocław, Elsevier Urban & Partner [in Polish]
61. Brotas AM, Cunha JM, Lago EH, Tumor necrosis factor-alpha and the cytokine network in psoriasis: An Bras Dermatol, 2012; 87(5); 673-81
62. Shi HJ, Zhou H, Ma AL, Oxymatrine therapy inhibited epidermal cell proliferation and apoptosis in severe plaque psoriasis: Br J Dermatol, 2019; 181(5); 1028-37
63. Kokolakis G, Giannikaki E, Stathopoulos E, Infliximab restores the balance between pro- and anti-apoptotic proteins in regressing psoriatic lesions: Br J Dermatol, 2012; 166(3); 491-97
64. Yu Q, Tong Y, Cui L, Efficacy and safety of etanercept combined plus methotrexate and comparison of expression of pro-inflammatory factors expression for the treatment of moderate-to-severe plaque psoriasis: Int Immunopharmacol, 2019; 73; 442-50
65. Elango T, Thirupathi A, Subramanian S, Methotrexate treatment provokes apoptosis of proliferating keratinocyte in psoriasis patients: Clin Exp Med, 2017; 17; 371-81
Figures
 Figure 1. Apoptosis trials: intrinsic and extrinsic (TNF-alpha – tumor necrosis factor alpha; TNFR – tumor necrosis factor alfa receptor; FasL – Fas ligand; TRADD – TNFRSF1A Associated Via Death Domain; FADD – Fas-associated protein with death domain).
Figure 1. Apoptosis trials: intrinsic and extrinsic (TNF-alpha – tumor necrosis factor alpha; TNFR – tumor necrosis factor alfa receptor; FasL – Fas ligand; TRADD – TNFRSF1A Associated Via Death Domain; FADD – Fas-associated protein with death domain). Figure 2. Autoimmune diseases based on apoptosis.
Figure 2. Autoimmune diseases based on apoptosis. Figure 3. Apoptosis in psoriasis mainly regulated by Bcl-2 family proteins (BH1-BH4 – proteins domains; TM – transmembrane domain).
Figure 3. Apoptosis in psoriasis mainly regulated by Bcl-2 family proteins (BH1-BH4 – proteins domains; TM – transmembrane domain). In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952









![Effects of TNF-α [60].](https://jours.isi-science.com/imageXml.php?i=t1-medscimonit-26-e922035.jpg&idArt=922035&w=1000)